Ester Formation and Hydrolysis during Wet–Dry Cycles: Generation of Far-from-Equilibrium Polymers in a Model Prebiotic Reaction
Irena Mamajanov, Patrick J. MacDonald, Jingya Ying, Daniel M. Duncanson, Garrett R. Dowdy, Chelsea A. Walker, Aaron E. Engelhart, Facundo M. Fernández, Martha A. Grover, Nicholas V. Hud, and F. Joseph Schork
Macromolecules, 2014, 47 (4), pp 1334–1343. doi: 10.1021/ma402256d.
Publisher | ResearchGate | Google Scholar
Scientific Abstract
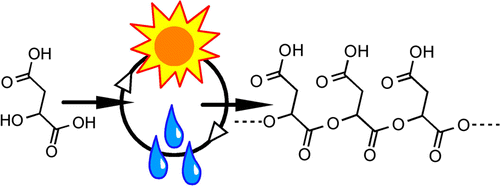
Biopolymers exist within living cells as far-from-equilibrium metastable polymers. Living systems must constantly invest energy for biopolymer synthesis. In the earliest stages of life on Earth, the complex molecular machinery that contemporary life employs for the synthesis and maintenance of polymers did not exist. Thus, a major question regarding the origin of life is how the first far-from-equilibrium polymers emerged from a prebiotic “pool” of monomers. Here, we describe a proof-of-principle system, in which l-malic acid monomers form far-from-equilibrium, metastable oligoesters via repeated, cyclic changes in hydration and temperature. Such cycles would have been associated with day–night and/or seasonal cycles on the early Earth. In our model system, sample heating, which promotes water evaporation and ester bond formation, drives polymerization. Even though periodic sample rehydration and heating in the hydrated state promotes ester bond hydrolysis, successive iterations of wet–dry cycles result in polymer yields and molecular weight distributions in excess of that observed after a single drying cycle. We term this phenomenon a “polymerization ratchet”. We have quantitatively characterized the “ratchet” of our particular system. Ester bond formation rates and oligoester hydrolysis rates were determined for temperatures ranging from 60 to 95 °C. Based on these rates, a mathematical model was developed using polycondensation kinetics, from which conditions were predicted for oligoester growth. This model was verified experimentally by the demonstration that l-malic acid monomers subjected to multiple wet–dry cycles form oligoesters, which reach a steady-state concentration and mean length after several cycles. The concentration of oligoesters that persist between subsequent steady-state cycles depends on the temperature and durations of the dry and wet phases of the cycle. These results provide insights regarding the potential for very simple systems to exhibit features that would have been necessary for initiation of polymer evolution, before the emergence of genomes or enzymes.
Lay Abstract
Almost all the polymers found in life (proteins, DNA/RNA, and some carbohydrates) are formed by chemical reactions where single molecule monomers combine into polymers, with each linkage generated between the monomers being produced with the loss of water. In this paper, we examined experimentally a scenario many have invoked as helping encourage that loss of water: day/night cycles. We found that a model monomer, called malic acid, when subjected to hot and dry/cool and wet cycles, like those expected to be produced by the sun rising and setting, could assemble into polymers. We found that the wet cycles had to be cool enough to not degrade the polymer produced back into monomer – under hot, wet conditions, malic acid polymers degraded back into malic acid monomers. Through computer simulations and experiment, we mapped out the temperatures where this happened. Given the right conditions, each hot, dry cycle produced more monomer than was lost in each cool, wet cycle. This map of temperatures provides some indications about the environment in which day/night cycles could have induced the earliest polymers to form. We called this a “polymerization ratchet.”