Intercalation as a means to suppress cyclization and promote polymerization of base-pairing oligonucleotides in a prebiotic world
Eric D. Horowitz, Aaron E. Engelhart (Shared first author), Michael C. Chen, Kaycee A. Quarles, Michael W. Smith, David G. Lynn, and Nicholas V. Hud
Proceedings of the National Academy of Sciences of the United States of America, 2010, 107 (12), pp 5288-5293. doi: 10.1073/pnas.0914172107
Publisher | ResearchGate | PubMed | Google Scholar
Featured in: Georgia Tech Press Release | NSF | New Scientist | RSC Chemistry World
Coauthor Michael Chen, who was my undergraduate mentee when I was a graduate student, and who is now in a joint PhD program between the University of Cambridge and the NIH, was featured here.
Scientific Abstract
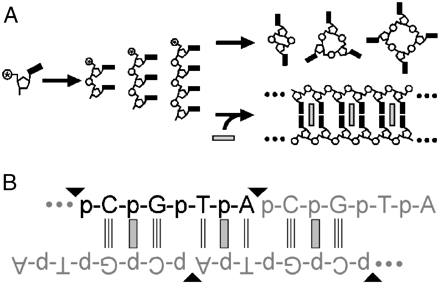
The RNA world hypothesis proposes that nucleic acids were once responsible for both information storage and chemical catalysis, before the advent of coded protein synthesis. However, it is difficult to imagine how nucleic acid polymers first appeared, as the abiotic chemical formation of long nucleic acid polymers from mononucleotides or short oligonucleotides remains elusive, and barriers to achieving this goal are substantial. One specific obstacle to abiotic nucleic acid polymerization is strand cyclization. Chemically activated short oligonucleotides cyclize efficiently, which severely impairs polymer growth. We show that intercalation, which stabilizes and rigidifies nucleic acid duplexes, almost totally eliminates strand cyclization, allowing for chemical ligation of tetranucleotides into duplex polymers of up to 100 base pairs in length. In contrast, when these reactions are performed in the absence of intercalators, almost exclusively cyclic tetra- and octanucleotides are produced. Intercalator-free polymerization is not observed, even at tetranucleotide concentrations > 10,000-fold greater than those at which intercalators enable polymerization. We also demonstrate that intercalation-mediated polymerization is most favored if the size of the intercalator matches that of the base pair; intercalators that bind to Watson–Crick base pairs promote the polymerization of oligonucleotides that form these base pairs. Additionally, we demonstrate that intercalation-mediated polymerization is possible with an alternative, non-Watson–Crick-paired duplex that selectively binds a complementary intercalator. These results support the hypothesis that intercalators (acting as ‘molecular midwives’) could have facilitated the polymerization of the first nucleic acids and possibly helped select the first base pairs, even if only trace amounts of suitable oligomers were available.
Lay Abstract
In this work, we consider one route by which the earliest genetic polymers might have assembled. When very short nucleic acid chains (such as those that might have been produced in early life) are base-paired in a double helix, they can be linked into longer, potentially useful, polymers. However, when these short chains are floating freely in solution, they tend to link at their ends – like a dog biting its own tail. Unfortunately, because they’re so short, short molecules tend not to base pair, making them especially subject to this type of circularization pathway. What we showed in this work is that the addition of a small molecule that binds to base-paired nucleic acids, called ethidium, promotes the assembly of even short (four bases) nucleic acids. When ethidium is present, these molecules link into long (~100 residue) polymers. When it is absent, they form the aforementioned circular species. Additionally, we found that the short chains have to have the right sequence. If we take molecules that can’t base pair (i.e., strands that try and do something other than A with T and G with C), they don’t form long polymers. We needed base pairing to get polymerization, showing that this small molecule could promote assembly of short chains of nucleic acids while still allowing for information transfer (i.e., copying) between these chains.