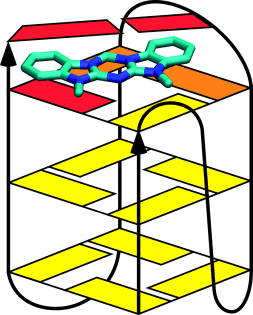
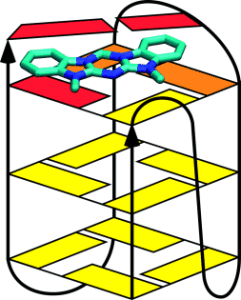
Submicromolar, Selective G-Quadruplex Ligands from One Pot: Thermodynamic and Structural Studies of Human Telomeric DNA Binding by Azacyanines
Özgül Persil Çetinkol, Aaron E. Engelhart (shared first author), Rupesh K. Nanjunda, W. David Wilson, and Nicholas V. Hud
ChemBioChem, 2008, 9 (12), pp 1889-1892. doi: 10.1002/cbic.200800234
Publisher | ResearchGate | PubMed | Google Scholar
Scientific Abstract
A molecular architecture with a shape that is slightly larger than a Watson–Crick base pair was investigated as a potential G-quadruplex ligand. Azacyanines, which possess this architecture, were found to bind on the end of the quadruplex stack (as shown in the figure) and to exhibit high selectivity over duplex binding. The chemical properties, ease of synthesis, and great potential for modification make the azacyanines and their analogues attractive for development as G-quadruplex ligands in drug development.
Lay Abstract
In addition to the well-known double helix formed by DNA, this molecule can also form a four-stranded structure called a G-Quadruplex. These structures are known to be associated with a number of biological processes, including the development of cancer. Drug molecules that can modulate the relative tendency for a strand of DNA to exist in a double helix versus a G-Quadruplex, therefore, are potential therapeutic agents in cancers. In this paper, we show that a molecule with a very easy method of synthesis can selectively bind a G-Quadruplex, with very low affinity for a DNA duplex (double helix). This low affinity for duplex DNA is particularly valuable, since much of the toxicity of chemotherapeutic drugs is related to their affinity for duplex DNA.